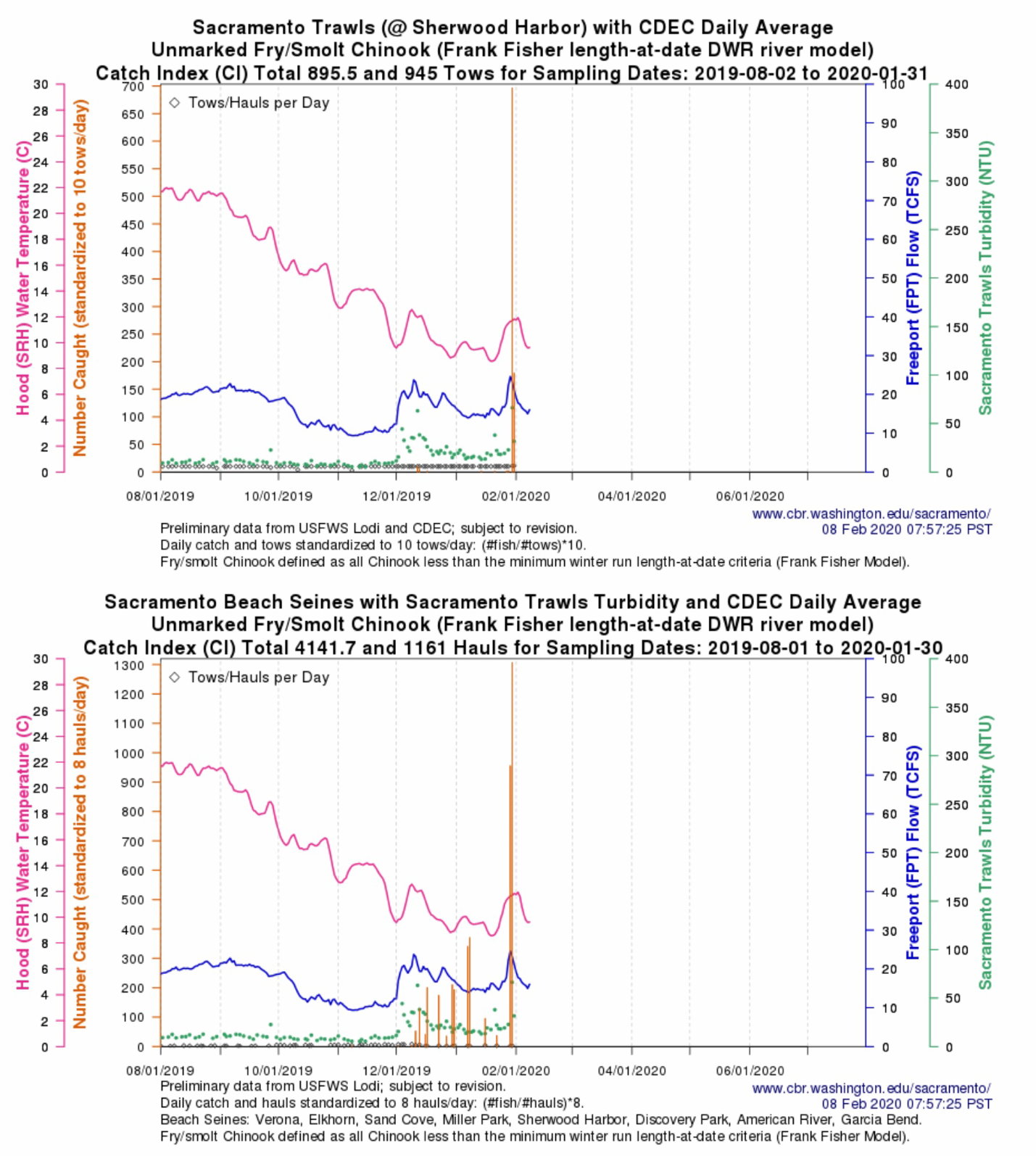
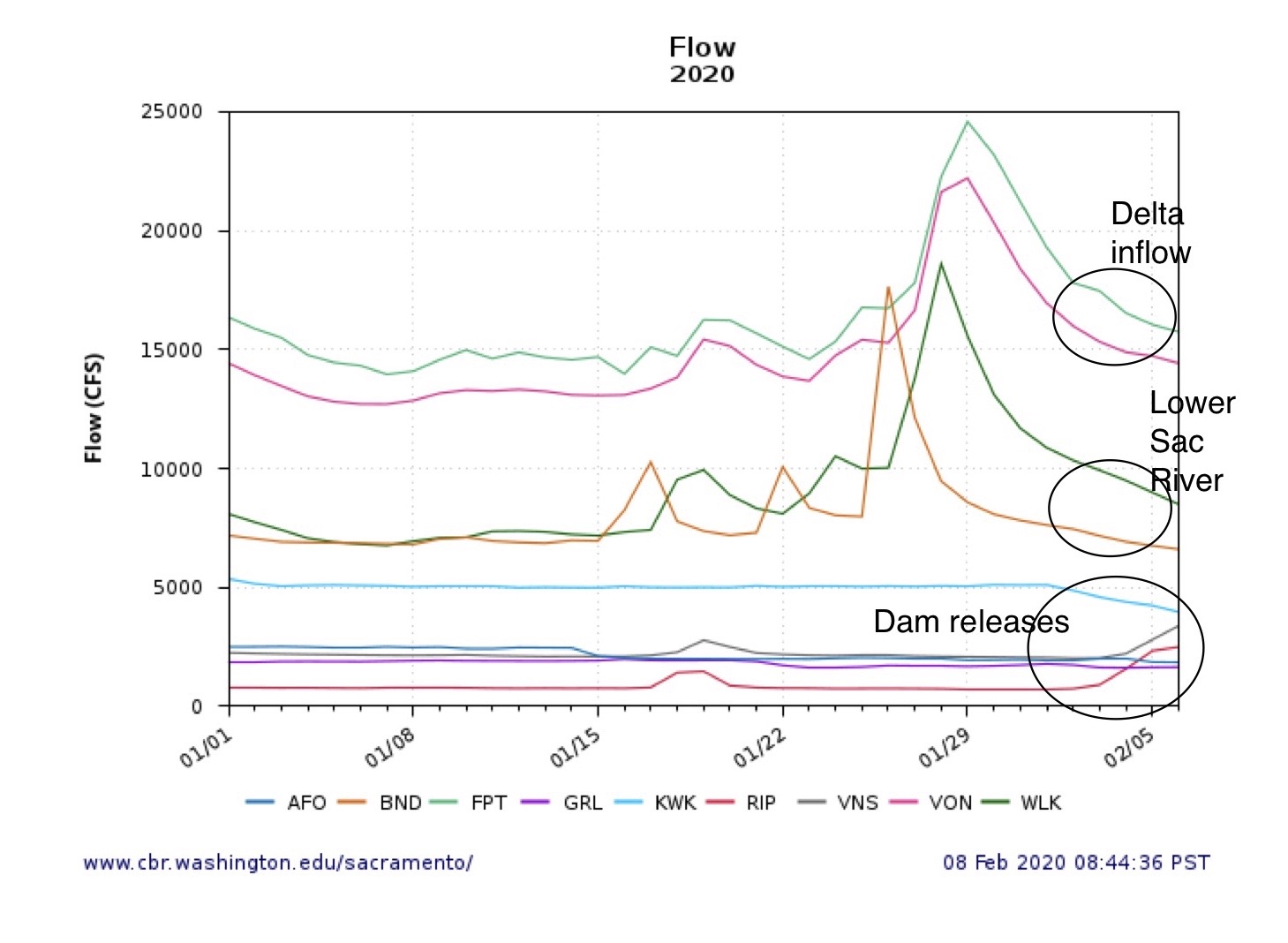
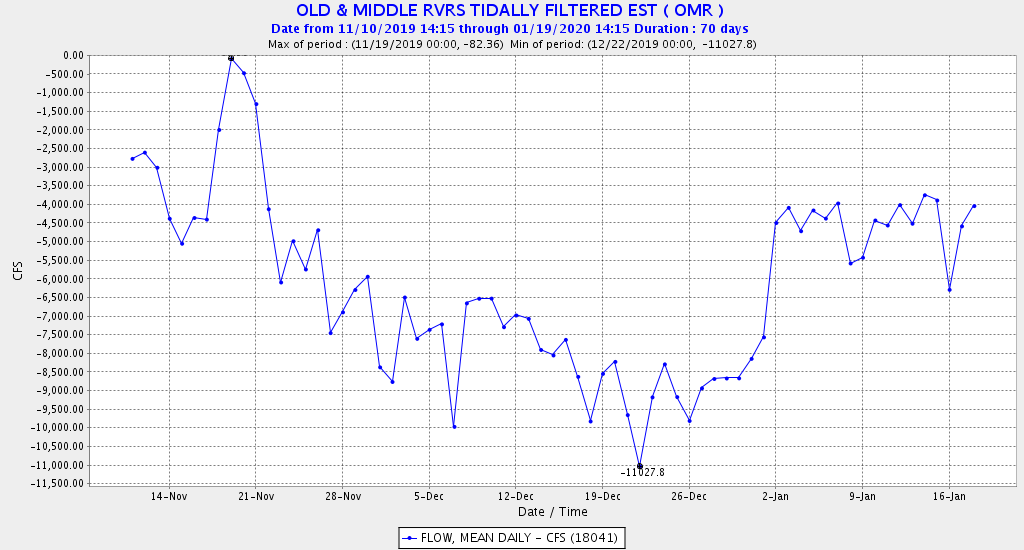
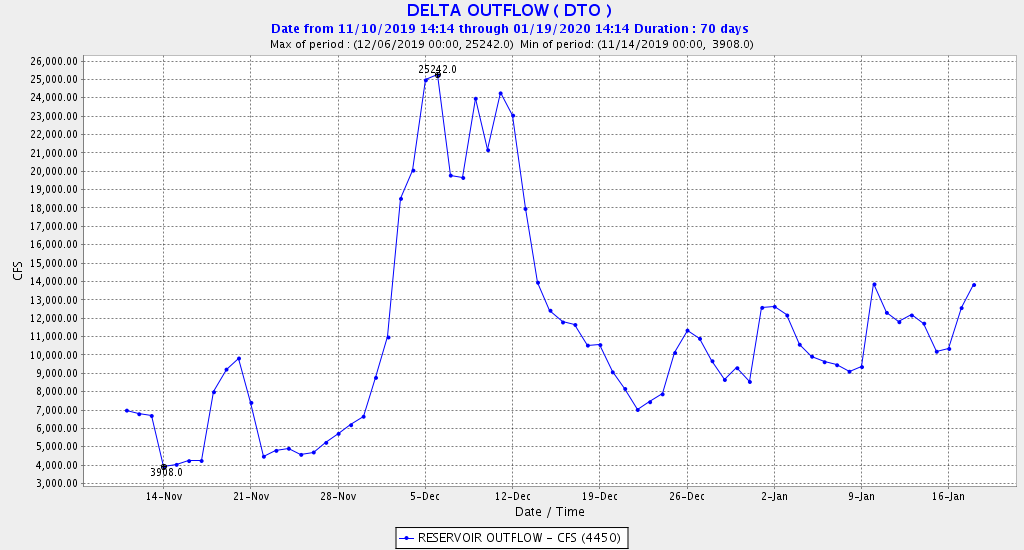
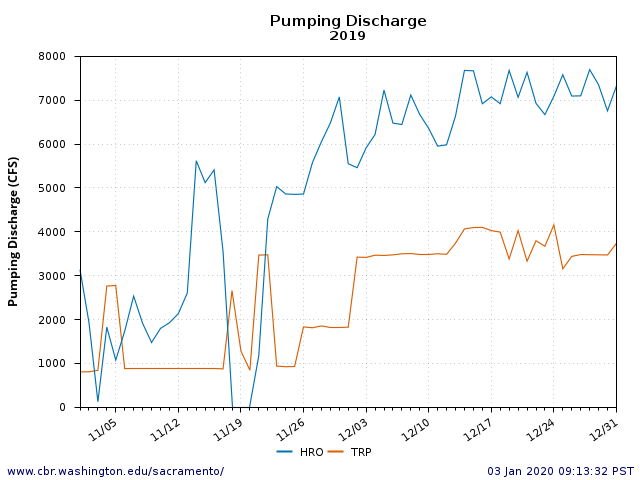
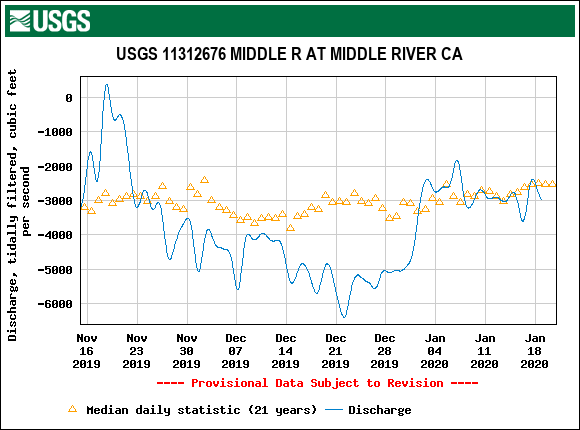
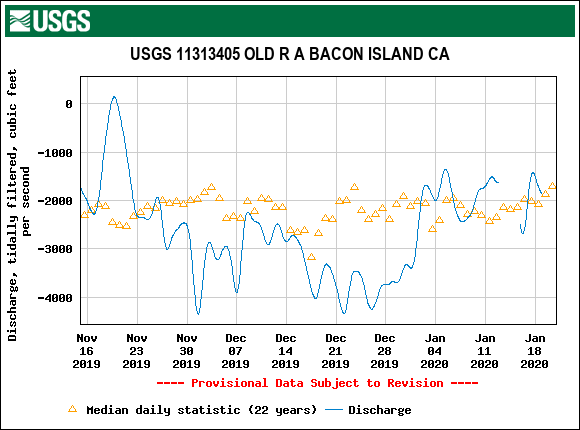
The Problem
Hatcheries bypass the high mortality life-history phases of wild salmon populations. As a result, hatcheries contribute far greater salmon smolt production to the ocean per number of eggs than do wild populations. Without hatcheries, the replacement rate of Central Valley salmon populations would be less than 1-to-1, and the populations would move toward extinction. Without hatcheries, there would be no commercial or sport salmon fisheries in California today.
But hatcheries bring many real problems for wild salmon. These problems include in-breeding/domestication, disease transmission, and over-harvest of, competition for, and direct and indirect predation on wild salmon populations. In-breeding has already had dramatic effects on the salmon populations, leading to the loss or degradation of many important life-history traits and of subpopulations that carry these traits (the “Portfolio Effect”).
Having lost many traits that nature provided over millions of years of natural selection, hatchery salmon today are simply less able to cope with the new world they now face. They mature younger and smaller. They are less able to adapt to changes in their food supply. They often can’t compete and are less able to avoid predators. Many arrive on spawning grounds too early, and others can’t find their natal streams. Their offspring are also far less capable of coping with the stress and adversities, including harvest, pollution, and habitat loss and degradation.
Over-harvest, competition, and straying of hatchery fish has led to the dominance of hatchery fish in the Central Valley salmon populations and homogenization among the populations. Some populations now survive only in hatcheries or in captive breeding programs.
The Solution
Many of elements of the problem have already occurred and are difficult to overcome. While some elements are irreversible, it is not too late to limit or reduce some of the negative effects. A comprehensive set of actions and strategies can avoid, minimize, mitigate, or even reverse these effects. These actions and strategies should include:
1. Reduce competition between hatchery and wild salmon in spawning, rearing, and migrating habitat.
- Do not allow hatchery salmon to spawn in prime wild salmon spawning areas. Sorting at weirs can preclude passing hatchery spawners if hatchery fish are all marked.
- Do not release hatchery juveniles into rearing and migrating habitats heavily used by remaining stocks of wild salmon. Programs throughout the range of Pacific coast salmon, including the Central Valley, now release hatchery smolts into net pens in rearing areas less frequented by wild salmon. The best fishery returns to the Central Valley have been from smolts released from coastal net pens.
2. Reduce straying of hatchery origin spawners into other spawning rivers.
- Barge hatchery smolts to reduce competition and predation on wild juvenile salmon and decrease the straying of adults that results from trucking. Barging can help imprint smolts on home rivers and hatcheries.
- Monitor and sort adult salmon returns in rivers and hatcheries to further eliminate straying.
- Focus more hatchery production on rivers and streams that do not support significant wild salmon.
3. Increase harvest of hatchery salmon, while reducing harvest of wild salmon.
- Focus harvest on hatchery stocks to help protect wild stocks. Release hatchery smolts into locations that focus harvest of adults in areas not frequented by wild salmon. Adult hatchery salmon tend to stay in or return to areas where smolts were released.
- Increase existing efforts to reduce the mixed-stock harvesting problem by reducing mixed-stock fishery exploitation rates to levels that are sustainable by wild stocks. Promote selective harvest of hatchery fish by permitting sport fishermen to retain only hatchery fish or to retain more hatchery fish than wild fish. This would require marking most or all hatchery smolts.
4. Improve disease control.
- Hatchery fish experience greater susceptibility to infectious diseases due to higher rearing densities, higher levels of stress and poorer water quality. Diseases/infections can be spread to wild population elements, though research is needed to determine the extent of this threat.
- Improve filtration systems at hatcheries to reduce the disease threat. This will also alleviate concerns about reintroducing salmon and steelhead upstream of hatcheries.
5. Improve the genetic makeup of hatchery (and wild) salmon
- Reverse engineer aspects of genetic diversity that has been selected out. Preferentially spawn 4-5 year-old adults at hatcheries. Diversify timing of adult runs by breeding hatchery fish throughout the spawning run. The Mokelumne Fish Hatchery is already implementing many such practices. “Bad alleles can be purged.”
- Use conservation hatchery actions to enhance the genetic diversity and fitness to help recover depleted wild populations.
- Use more wild fish for hatchery broodstocks, particularly fish with more favorable traits.
- Do not allow adult hatchery fish into spawning habitat used by wild fish.
- Be more selective in choosing spawners for hatcheries.
- Develop and support pure strains of wild salmon above dams through trap and haul programs.
- Promote populations and subpopulations that protect or increase diversity (improve the Portfolio).
- Develop captive stocks with desired natural traits – with less genetic drift, inbreeding and domestication,
- Increase monitoring, research, experimentation, and adaptive management on the extent and consequences of domestication selection, as well as steps that may be taken to reduce its effects.
- Evaluate and operate each hatchery program independently to address its program and its contribution to the overall problem.
Conclusion
Wild salmon populations in California’s Central Valley are already compromised to various degrees by hatchery salmon, over-harvest, and habitat degradation. More can be done to protect wild salmon production and minimize the threat from hatcheries, while continuing to provide valuable commercial and sport fisheries supported by hatcheries. We can save our salmon and eat them too.
For a more comprehensive scientific review of these subjects see Sturrock et al. 2019 and Nash et al. 2007.